MY EVIDENCE.
MY CHOICE.
MYOBLOC.

MYOBLOC® is the first FDA-approved neurotoxin for the treatment of cervical dystonia (abnormal head position/neck pain) and a proven botulinum toxin B. Explore the evidence behind its strong legacy that extends back to 2000.
Jump to Section
collapseHow cervical dystonia can impact your patients
Click the play button to hear Dr. Bahroo discuss the challenges his patients have faced with cervical dystonia and what MYOBLOC has done for them.
Dr. Bahroo is a Movement Disorder Specialist and MYOBLOC prescriber based out of Washington, DC.
The potential negative impact of cervical dystonia on patients is significant*
Cervical dystonia may lead to:
Up to 75% of patients with cervical dystonia suffer from neck pain.9
Nearly 7 out of 10 patients report their pain as being moderate or severe.5
Quality of life scores have been reported to be comparable to those in Parkinson’s disease and stroke.8
*There are no studies showing that treating cervical dystonia affects all of the specific outcomes on this page.
MYOBLOC has a distinct mechanism of action10

Stage 1: MYOBLOC binds to receptors on the neuronal surface via the heavy chain.
Stage 2: MYOBLOC enters the nerve cell via endocytosis and is contained in vesicles.
Stage 3: MYOBLOC light chain moves to the cytosol where it cleaves synaptic vesicle-associated membrane protein (VAMP)* essential for acetylcholine (ACh) release.
*VAMP is a component of the protein complex responsible for docking and fusion of the synaptic vesicle to the presynaptic membrane, a necessary step in neurotransmitter release.
For cervical dystonia, blocking ACh release inhibits muscle contraction and allows the injected muscle to assume a more normal tone.10

Why consider MYOBLOC?
Click the play button to hear some of the things Dr. Bahroo considers when determining if MYOBLOC will be a good fit for his patients.
Proven clinical efficacy and safety for cervical dystonia
MYOBLOC has been evaluated in clinical studies for a range of adult patients with cervical dystonia.
SELECT A DATA SET
-
A randomized, double-blind, multicenter, placebo-controlled, 16-week trial
A randomized, double-blind, multicenter, placebo-controlled, 16-week trial
- 109 patients enrolled
- 3 dose arms: 5,000 Units (n=36) or 10,000 Units (n=37) vs. placebo (n=36)
- TWSTRS-total score at Week 4: Primary endpoint
- TWSTRS-pain score at Week 4: Tertiary endpoint
-
A randomized, double-blind, multicenter, placebo-controlled, 16-week trial
A randomized, double-blind, multicenter, placebo-controlled, 16-week trial
- 77 patients enrolled
- 2 dose arms: 10,000 Units (n=39) vs. placebo (n=38)
- TWSTRS-total score at Week 4: Primary endpoint
- TWSTRS-pain score at Week 4: Tertiary endpoint
-
A randomized, double-blind, multicenter, noninferiority trial
A randomized, double-blind, multicenter, noninferiority trial
- 93 patients new to treatment with botulinum toxins were analyzed
- 2 dose arms: BOTOX 150 Units (n=47) or MYOBLOC 10,000 Units (n=46)
- TWSTRS-total score 4 weeks post-injection: Primary endpoint
- TWSTRS-pain score at Week 4: Secondary endpoint
-
- 427 patients enrolled
- 2 dose arms: 5,000 Units (toxin-naive patients who previously received placebo) or 10,000 Units, or the highest MYOBLOC dose previously received
- Long-term safety and tolerability: Primary endpoint
- TWSTRS-total score: Secondary endpoint
Significant clinical efficacy in both Toxin A-responsive and -resistant cervical dystonia10-12
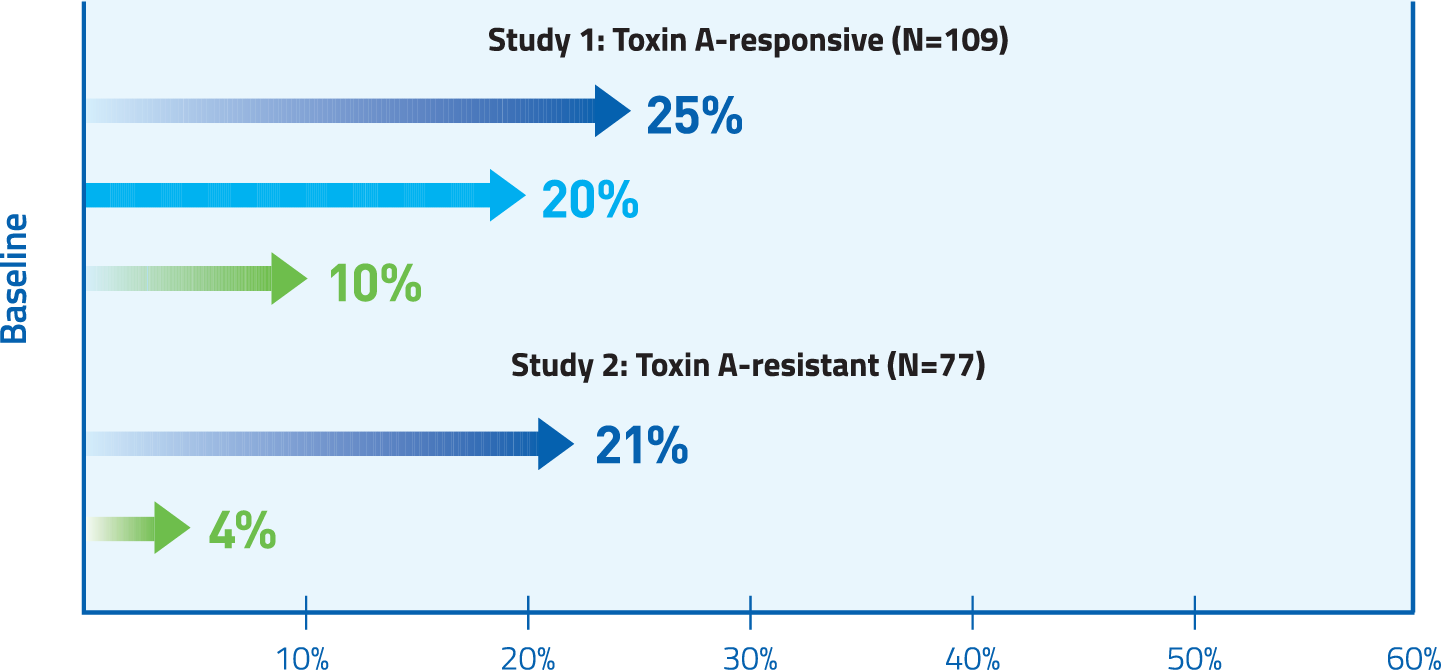
At Week 4 in both Toxin A-responsive and Toxin A-resistant patients, MYOBLOC significantly improved TWSTRS-total score.

At Week 4 in both Toxin A-responsive and Toxin A-resistant patients, MYOBLOC significantly improved TWSTRS-total score.
Mean change from baseline was 4.3 and 2 for placebo (Study 1 and Study 2, respectively); 9.3* for 5,000 U (Study 1), and 11.7* and 11.1* for 10,000 U (Study 1 and Study 2, respectively) * P <0.05 vs. placebo.
Abbreviation: TWSTRS, Toronto Western Spasmatic Torticollis Rating Scale.
Early and significant pain reduction10-13
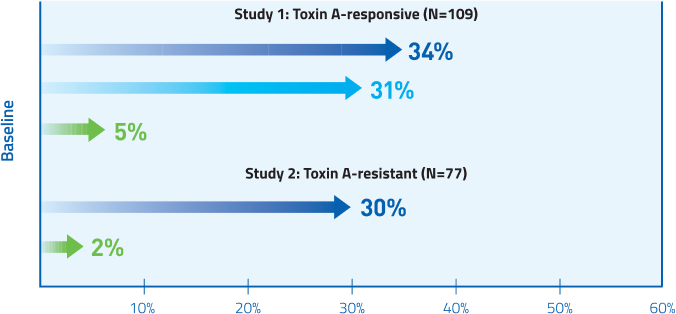
MYOBLOC provided significant pain reduction for both Toxin A-responsive and Toxin A-resistant patients at Week 4

MYOBLOC provided significant pain reduction for both Toxin A-responsive and Toxin A-resistant patients at Week 4
Mean change from baseline in TWSTRS-pain score
Study 1, Week 4: Placebo 0.5; 5,000 U 3.6†; 10,000 U 4.2.†
Study 2, Week 4: Placebo 0.2; 10,000 U 3.6.†
†P<0.005 vs. placebo.
Study 1, Week 2: Placebo 0.88; 5,000 U 4.12; 10,000 U 4.03.
Study 2, Week 2: Placebo 0.24; 10,000 U 2.54.
In an exploratory analysis, MYOBLOC showed reduction of TWSTRS-pain score for both Toxin A-responsive and Toxin A-resistant patients at Week 2.
Although there was a MYOBLOC-associated decrease in pain, there remained many patients who experienced an increase in dystonia-related neck pain irrespective of treatment group.
Post-hoc and exploratory analyses are descriptive in nature; statistical significance can not be derived from the results.
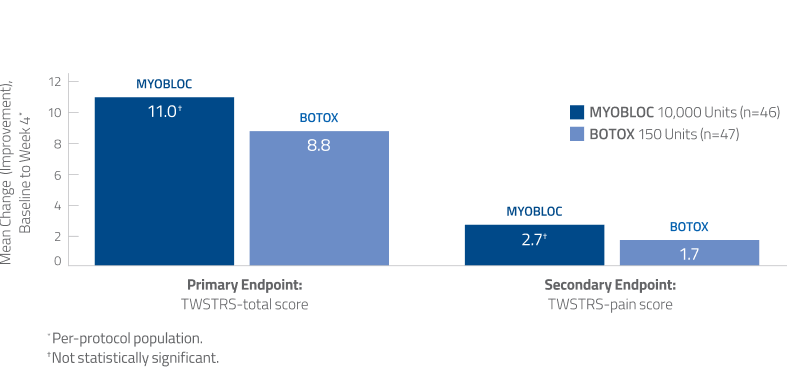
Noninferiority of MYOBLOC vs. BOTOX was established in a head-to-head study14
Additional Data
- TWSTRS-total score and TWSTRS-pain scores were numerically greater with MYOBLOC than BOTOX, but this difference was not statistically significant
- No significant difference in median duration of clinical effect
Using TWSTRS-total score, clinically meaningful improvement has been established for botulinum Toxin A (point difference: 7, minimal improvement; >8, much improved; >10, very much improved).
Pain relief maintained over 3 years13,15
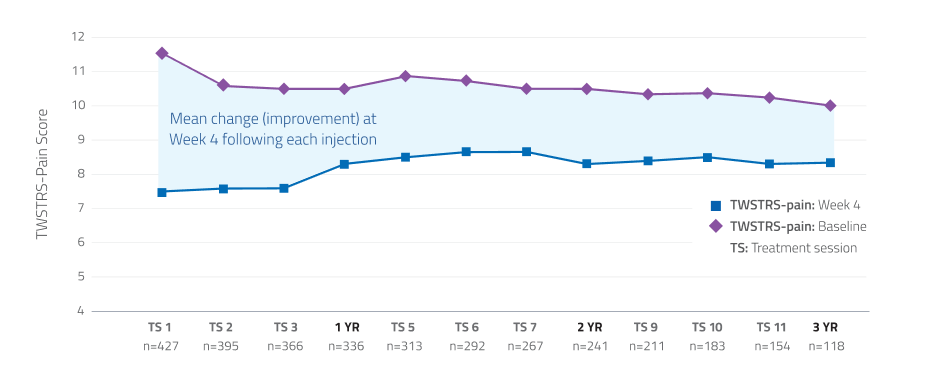
Additional Data:
In a long-term open-label study, patients demonstrated consistent improvement in TWSTRS-pain scores through 12 treatment sessions.*
*Limitations associated with open-label study design, include lack of comparator arm, decreasing sample size and potential continued involvement of responders and attrition of nonresponders.
MYOBLOC has demonstrated tolerability10
Studies 1, 2, and 4*
Most commonly reported adverse reactions in >5% of MYOBLOC-treated patients at any dose and >5% more common than placebo
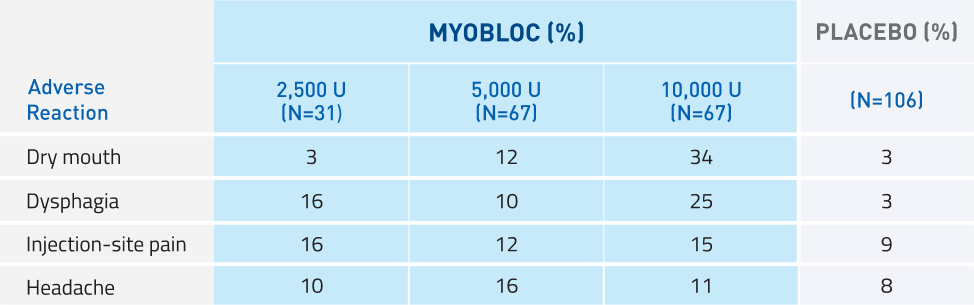
*Study 4: A randomized, double-blind, multicenter, placebo-controlled, 16-week trial to determine the safety and efficacy of MYOBLOC in both Toxin A-responsive and Toxin A-resistant patients with CD; 122 patients enrolled across 4 dose arms: 2,500 Units (n=31), 5,000 Units (n=31), or 10,000 Units (n=30) vs. placebo (n=30).
Safety profile comparable to BOTOX†,14

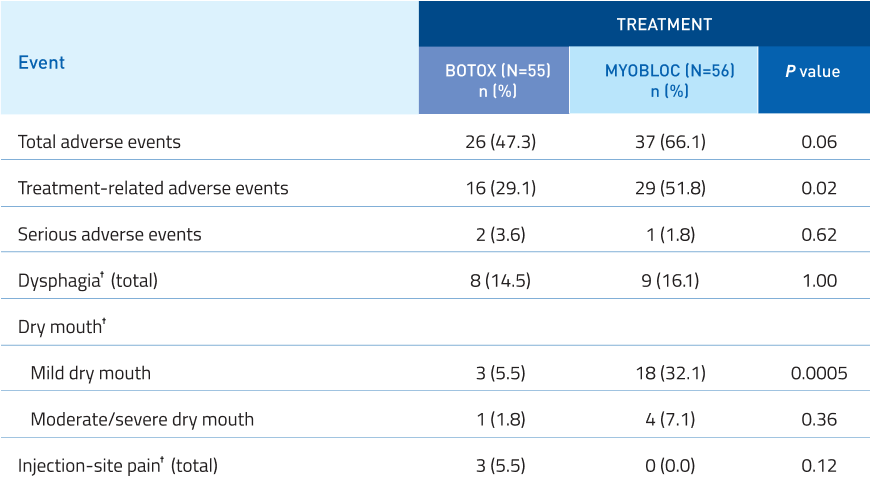
Additional Data:
No significant difference in serious adverse events.
Mild dry mouth was increased with MYOBLOC.
Dysphagia, moderate/severe dry mouth, and
injection-site pain were similar to BOTOX.
†Categories of interest have been defined as “dry mouth”, “dysphagia”, and “injection-site pain” because they represent frequent adverse events that have been
associated with both botulinum toxin serotypes; treatment-related incidence is the same as overall incidence for these events. No other treatment-related adverse
events occurred in >10% in either BOTOX or MYOBLOC in the intent-to-treat population.
‡Fisher’s exact test (BOTOX vs. MYOBLOC).
Take the next step with MYOBLOC
-

Order MYOBLOC
Order MYOBLOCOrder by phone or email and we'll help you get the treatment your patients need.
-

Get Live Support
Request A SpecialistGet in touch with a Reimbursement Specialist who can help you navigate patient support services, insurance benefits, and more.
-

Access Resources
View ResourcesFind resources that can help guide you through the process of injecting MYOBLOC.
