Order MYOBLOC
by phone or email


We’re standing by to help you get the treatment your patients need. Call or click to get in touch with us and we’ll make ordering as simple as possible.
-

Call 1-888-461-2255
MYOBLOC Premier Partners can enjoy special access to our volume-based pricing model. The more MYOBLOC you order, the more you save. Ask about it today. -

Email your order to GMB-SPS-SOLSTICE@cordlogistics.com
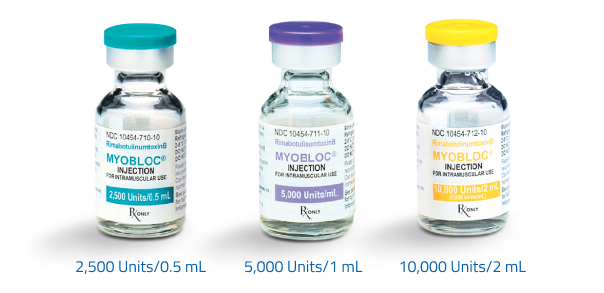
Include your name, your MYOBLOC account number, the number of vials and vial sizes you would like, and where you would like them shipped. Our team will reach out to confirm your order.